Rates of congenital syphilis—infections passed from parent to infant during pregnancy or childbirth—have been skyrocketing in the United States in the last few years. At the same time, the country is facing a severe shortage of the only antibiotic that has been approved to treat syphilis in pregnant women. A new survey of health care providers by the National Coalition of STD Directors (NCSD) has found that this shortage is indeed delaying care for pregnant patients.
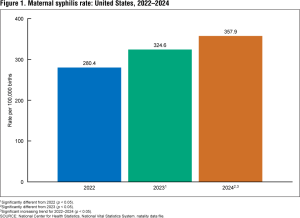
CDC data show that more than 3,700 babies were born with syphilis in 2022—more than 10 times the number born in 2012. These infections can lead to miscarriage, stillbirth, prematurity, low birthweight, or death shortly after birth. Treatment during pregnancy can help both parent and child, but the shortage of Bicillin-L-A is making that very difficult.
The Drug Shortage
Bicillin L-A, which is also called penicillin G benzathine, is the preferred treatment for primary and secondary (P&S) syphilis all populations. It is the only treatment approved for pregnant people. There have been shortages of this drug before—first between 2005 and 2010 and again in 2017 as congenital syphilis cases became more common. Pfizer is currently the only pharmaceutical company that makes this medication. It announced a manufacturing delay in June. The company said shortages would likely go on through 2024.
In the meantime, the CDC is suggesting that clinicians prioritize Bicillin L-A for pregnant people with syphilis and babies with congenital syphilis. All other patients should get doxycycline.
The Surveys
In late August, the National Coalition of STD Directors (NCSD) surveyed more than 100 sexual health clinics to determine how the drug shortage was affecting them and their patients. It found that in the three months prior to the survey, 40% of clinics had attempted to order Bicillin L-A only to be told it was not available. In addition, 28% of clinics said they had to get the drug from a nearby clinic or refer the patients elsewhere for treatment.
NCSD sent out another survey in early November and got responses from 151 clinics and 136 health departments. Respondents represented 39 states, one territory, Washington, D.C., and communities served by Indian Health Services. The results show that the drug shortage is worsening, and that patient care is being impacted.
Respondents were asked about the three months prior to the survey. Results included:
- 46% of clinics have attempted to order Bicillin L-A, but the drug was not available (this is up from 40% in the first survey conducted in August)
- 40% of clinics have had their Bicillin L-A orders delayed (this was up from 37% in August)
- 62% percent of clinics are currently reserving doses for pregnant people and other patients who cannot use doxycycline
- 10% of clinics are already referring patients to other clinics for syphilis treatment and another 23% of clinics anticipate needing to divert patients before the shortage is resolved
Health department respondents from 13 different states and one Indian Health Services agency say they’ve received reports of a pregnant person in their jurisdiction who was unable to access Bicillin L-A (All of these pregnant patients did receive treatment, but there were delays of up to 28 days in getting that treatment.)
Only 13% of clinic respondents believe Pfizer’s assurances that the Bicillin L-A shortage will be resolved in the first half of 2024
To control the rising rates of congenital syphilis and prevent illness and death among infants, pregnant people need access to screening and treatment. If this drug shortage does not end soon congenital syphilis rates will likely get even worse.