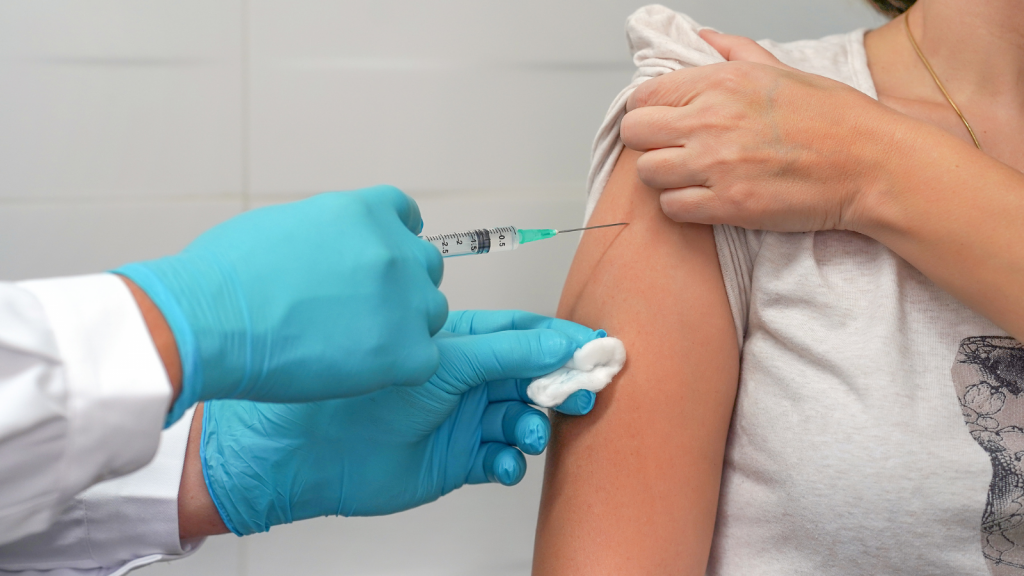
Gilead Sciences announced remarkable results in a Phase III PrEP trial. The trial tested whether a drug—lenacapavir—could be used as Pre-Exposure Prophylaxis (PrEP) to prevent HIV infection. The results found no infections in women who were given lenacapavir injections two times per year.
PrEP is a prevention strategy that involves using a prescription medicine to reduce the risk of new HIV infection. The CDC recommends PrEP for those at high risk of getting HIV. PrEP has been found to be 99% effective in preventing sexually transmitted HIV when used correctly. It is a little less effective (about 74%) at preventing HIV in people who use injection drugs.
There are currently three medications approved for use as PrEP in the United States. Truvada and Descovy are pills that need to be taken daily. (The two brands are made by different manufacturers but contain similar active ingredients.) There is also a shot given every two months called Apretude which uses a different active ingredient. Truvada and Apretude are approved for men and for people who have receptive vaginal sex (cisgender women or transgender men). Descovy, however, has not been approved for those having vaginal sex.
The Purpose Study tested if lenacapavir, a drug already used to treat HIV, could be used as PrEP when given as an injection just twice a year.
The Purpose 1 Study
This first part of the Purpose study enrolled 5,338 women in South Africa and Uganda. All of the women in the study received some version of PrEP. Since existing methods of PrEP work so well, it would be unethical for the study to have a control group that took no medication at all.
Researchers divided participants into three groups:
- 2,134 women got twice-yearly lenacapavir shots
- 2,136 women took daily Descovy pills
- 1,068 women took daily Truvada pills
So that neither researchers nor participants knew who was getting which treatment, everyone who took Descovy or Truvada daily was given a twice-yearly placebo shot. All participants getting lenacapavir shots also took daily placebo pills.
There were 16 HIV infections among those taking Truvada, 39 among those taking Descovy, but zero among people getting lenacapavir.
To compare these results with people not taking any form of PrEP, researchers matched participants to local women with similar attributes. They tested these women using an HIV test that can determine if someone is HIV positive and whether the infection was acquired recently. The analysis from these women was referred to as the background HIV incidence.
The background HIV incidence rate was 2.4 infections per 100 women per year or 2.4%. In comparison, the incidence rate for the various PrEP drugs was 0% for lenacapavir, 1.7% for Truvada, and 2.0% for Descovy.
The results for lenacapavir were so good that the independent Data Monitoring Committee recommended Gilead stop the blinded phase of the trial and offer the injection to all participants.
Additional Analysis Needed
A complete report of these results has not been published yet. Gilead is planning to release more information at a future conference.
The initial results seem to suggest that oral PrEP medications did not work very well for this population. In fact, Descovy was not significantly better than no PrEP at all. Gilead, which also makes Descovy, said they will also look at whether women in this study took the required medication every day. Previous studies have found that cisgender women in lower-income countries are less likely than others to adhere to these instructions. Experts think this may be the result of the stigma. They can face such stigma from even having the medication in their possession.
Lillian Mworeko, an HIV advocate in Eastern Africa, told the New York Times: “For a young woman who can’t get to an appointment at a clinic in a town, a young woman who can’t keep pills without facing stigma or violence — an injection just twice a year is the option that could keep her free of HIV.”
Studying Lenacapavir in Other Populations
There are four other arms of the Purpose study that are investigating lenacapavir in different populations. Purpose 2 is studying the drug for use by cisgender men, transgender women and men, and non-binary individuals who have sex with partners assigned male at birth. Purpose 3 is looking at its use in cisgender women in the United States. Purpose 4 is investigating whether lenacapavir works to prevent HIV infection among injection drug users. And, at the request of European regulators, Gilead also announced a Purpose 5 that will take place there.
We can expect results of these studies to continue to roll in. Hopefully, they will be as promising.
Acting Like a Vaccine
Decades of research have failed to produce an HIV vaccine. But a PrEP shot that is 100% effective and only has to be taken twice a year could serve the same function.
Dr. Linda-Gail Bekker, an investigator in the trial, told the Times that reading the results gave her “cold shivers.” She added. “After all our years of sadness, particularly over vaccines, this truly is surreal.”
Concerns About Price
In the United States, lenacapavir is available as treatment for those who are living with HIV. It costs $42,500 per year.
Gilead says is willing to share its intellectual property with generic drug manufactures in lower-income markets for a licensing fee sooner rather than later. Still, it can take these manufacturers years to ramp up production. This also won’t help bring down the cost of the drug in the United States.
HIV advocates are calling on Gilead to come up with a bold plan for reducing the price of this medication in the U.S. and elsewhere.
What’s Next?
Gilead must replicate these results in one more trial among women before it can ask the FDA to approve lenacapavir for use as PrEP in this population. If it goes well, it might be available by the end of 2025.