
Non-Hormonal Contraception Options
Non-hormonal contraceptive methods fall into a few categories. These include barrier methods and surgical options.

Nearly all women use birth control at some point in their lives, and soon they’ll have one more option available. On May 22, the U.S. Food and Drug Administration (FDA) approved Phexxi, a non-hormonal contraceptive gel that works by keeping the pH levels in the vagina acidic, and thus inhospitable to sperm.
What makes this new option different? As mentioned, it will offer women a new non-hormonal option, so women who can’t or prefer not to use birth control with hormones will have an alternative to condoms, the copper IUD, diaphragm or cervical cap. It’s also an on-demand option, meaning it can be used as needed. The gel comes in an applicator and can be inserted just before sex.
Phexxi works by keeping the pH in the vagina too acidic for sperm to survive. You have to put a new applicator of Phexxi into the vagina every time you have sex. If it’s been an hour or more since you inserted the gel and you haven’t had sex yet, you need to put a new applicatorful in before you do.
If you use Phexxi perfectly, it’s 93% effective. In real life situations, however, it’s been found to be about 86% effective.
In clinical trials, some women developed cystitis, pyelonephritis (kidney infection) and other upper urinary tract infection (UTI), so it’s not recommended for women with a history of recurrent urinary tract infection or urinary tract abnormalities.

Non-hormonal contraceptive methods fall into a few categories. These include barrier methods and surgical options.

Many methods of birth control that are available today rely on hormones like those that our bodies make naturally. Hormonal methods come in many different forms—from pills to patches to shots—but all of them essentially work the same way.
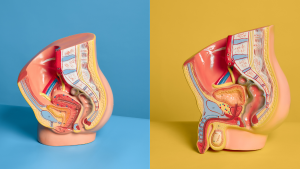
Our reproductive health is an important part of our sexual health and our overall health. It includes our reproductive organs and our ability to get pregnant or get someone pregnant when we choose.
Anyone who is having penis-in-vagina sex runs the risk of getting pregnant every time they have sex. Even if it’s your first time. Even if you have your period. Even if it’s a full moon and Mercury is in retrograde.
Sexual anatomy typically refers to the both the external sexual organs, like the vulva and penis, and the internal organs involved in reproduction, like the uterus and seminal vesicle. Learn about this part of the body and how it works.
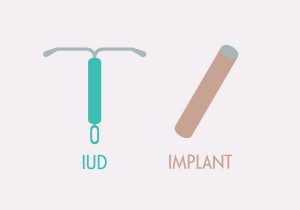
The name says it all. Long-acting reversible contraception, or LARC, is reversible birth control that provides long-lasting (think years) pregnancy prevention.
ASHA believes that all people have the right to the information and services that will help them to have optimum sexual health. We envision a time when stigma is no longer associated with sexual health and our nation is united in its belief that sexuality is a normal, healthy, and positive aspect of human life.
ABOUT
GET INVOLVED
ASHA WEBSITES
GET HELP
© 2025 American Sexual Health Association