
Antibiotic-Resistant Gonorrhea is On the Rise Globally
A new report from the World Health Organization (WHO) warns of rising levels of drug-resistant gonorrhea. The data comes from reported cases of gonorrhea in 12 countries across five WHO regions.
In late 2025, the US Food and Drug Administration (FDA) approved two new drugs to treat gonorrhea just weeks after the World Health Organization (WHO) sounded the alarm about antibiotic resistant infections. The two new drugs—gepotidacin and zoliflodacin—are both new kinds of antibiotics and represent the first completely new treatment options in over thirty years.
Gonorrhea is one of the most common bacterial sexually transmitted infections (STIs) in the world with an estimated 82 million cases across the globe each year. In the United States, the Centers for Disease Control and Prevention (CDC) estimate 1.6 million cases of gonorrhea each year.
Many people who get gonorrhea won’t have any symptoms. This is one of the reasons gonorrhea is so common; people pass it without every knowing they had it. If left untreated, gonorrhea can cause health issues including infertility in both men and women.
Gonorrhea is still considered a curable STI, but the bacterium that causes it has continuously evolved and made treatment more difficult. It has become resistant to most of the antibiotics that we have developed including sulfanilamides, penicillins, tetracyclines, and fluoroquinolones. That left cephalosporins as the only effective treatment we had left. The current standard treatment is an injection of ceftriaxone (which is a cephalosporin). Some people are also given follow up dose(s) of oral azithromycin though current guidelines recommend just the shot.
These new antibiotics are not part of any of the existing classes of antibiotics, and both of them are given orally which makes distribution much easier.
Gepotidacin will be sold under the name Blujepa. It was approved in March for treating urinary tract infections and has just been approved for gonorrhea as well. It comes in pill form. A standard course of treatment is eight pills taken in two doses.
In a study of 628 patients with gonorrhea, Bluejpa had similar results to the current standard treatment. Specifically, 93% of patients who took Blujepa were cured compared to 91% of patients who received a shot of ceftriaxone followed by one dose of azithromycin. Those who took Blujepa had more side effects, including diarrhea and nausea, but they were generally mild.
Zoliflodacin will be sold under the name Nuzolvence. The medication dissolves into water and is given as one dose. Research shows that it has similar cure rates. In a study of 930 patients with gonorrhea, 91% of patients who took Nuzolvence were cured at the one-week mark compared to 96% of patients who received the standard treatment.
Nuzolvence was developed by a partnership between the Global Antibiotic Research and Development Partnership—a nonprofit set up by the WHO—and the U.S.-based Innoviva Specialty Therapeutics. The goal of the nonprofit was to help encourage the development of new antibiotics as these drugs do not have the potential profit of other medical breakthroughs.
Nuzolvence was specifically developed to fight antibiotic resistance in gonorrhea and will only be used to treat gonorrhea. This is a deliberate strategy to prevent overuse which is one of the causes of antibiotic resistance.
Public health experts are very excited about these new drugs. Edward Hook, MD, an emeritus professor of medicine at University of Alabama and former ASHA board member, served as the protocol chair for studies of Nuzolvence. He told ASHA, “It’s been more than 30 years since the FDA approved a new antibiotic for gonorrhea treatment. At a time when antibiotic resistance is increasing worldwide, having new oral antibiotics effective against antibiotic resistant gonorrhea is a great addition to care for persons with an at risk for STIs.”

A new report from the World Health Organization (WHO) warns of rising levels of drug-resistant gonorrhea. The data comes from reported cases of gonorrhea in 12 countries across five WHO regions.
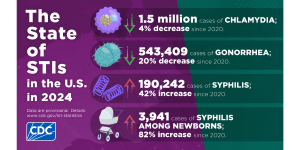
The CDC just released STI Surveillance Data for 2024 that show cases of chlamydia, gonorrhea, and syphilis are slowly declining.

On a recent episode of Love Island, a cast member sugested that we could blame our current STI epidemic on men who had sex with animals. She pointed to koalas with chlamydia as an example. There’s some truth here, but also a lot of misinformation.
There’s potential good news in gonorrhea prevention as a series of studies suggests that certain meningococcal B (MenB) vaccines can reduce the risk of gonorrhea.

Many people who take doxy PEP say it gives them peace of mind in their relationships and sex life. We sat down with Oscar Alexis, Efthimios, and Maxfield Haynes to talk about doxy PEP and get their perspective.

About 1.6 million cases of chlamydia and 600,000 cases of gonorrhea are diagnosed in the United Sates each year. A new study suggests that these patients may not be following through with STI treatment or getting the right antibiotics.

A clinical trial of a new antibiotic found that it works just as well as a current drug regimen for treating gonorrhea infections. This is important because the bacteria that causes gonorrhea has become resistant to most existing antibiotics.

The FDA just approved a new, fully at-home test for chlamydia, gonorrhea, and trichomoniasis. The tests, which is only for women, will be available without a prescription. Users can collect their own sample and have results in less than 30 minutes.
ASHA believes that all people have the right to the information and services that will help them to have optimum sexual health. We envision a time when stigma is no longer associated with sexual health and our nation is united in its belief that sexuality is a normal, healthy, and positive aspect of human life.
ABOUT
GET INVOLVED
ASHA WEBSITES
GET HELP
© 2026 American Sexual Health Association
We need to know if we can keep you company during this visit. We are useful for making this site work.
We use cookies to enhance your browsing experience. You can choose which cookies you want to accept.
Necessary cookies help make a website usable by enabling basic functions like page navigation and access to secure areas. The website cannot function properly without these cookies.
| Cookie | Provider | Purpose | Expiry |
|---|---|---|---|
digiconsent | This website | Stores your cookie consent preferences. | 1 year |
wordpress_logged_in_* | WordPress | Identifies logged-in users and their authentication details. | 14 days / Session |
wordpress_sec_* | WordPress | Stores authentication details for secure areas. | 14 days / Session |
wp-settings-* | WordPress | Stores user interface customization preferences. | 1 year |
wp-settings-time-* | WordPress | Stores the time when wp-settings cookie was set. | 1 year |
Analytics cookies help us understand how visitors interact with our website by collecting and reporting information anonymously. This helps us improve our website.
| Cookie | Provider | Purpose | Expiry |
|---|---|---|---|
_ga | Registers a unique ID to generate statistical data on website usage. | 2 years | |
_ga_* | Used by Google Analytics to store and count pageviews. | 2 years |
Marketing cookies are used to track visitors across websites. The intention is to display ads that are relevant and engaging for the individual user.
Functional cookies enable the website to provide enhanced functionality and personalization. They may be set by us or by third party providers.