A new fully at-home test for chlamydia, gonorrhea, and trichomoniasis is now available for online purchase, and users in certain major cities can even order it through DoorDash or Gopuff.
The Visby Medical Women’s Sexual Health Test, which won FDA approval in March, comes with its own testing device that plugs into an electrical outlet. Users also need to download the companion app which will provide instructions for collecting a vaginal sample, using the device, and uploading a picture of the results. The device processes a PCR test similar to those done in laboratories and offers results in 30 minutes.
The sexual health test—which is only for women—can be purchased on Visby’s website or through online health testing site Everlywell for $149.99. Most people will receive it in the mail, but users in 10 major cities, including San Francisco, Las Vegas, New York, and Atlanta, may be able to get same-day delivery through DoorDash or Gopuff. (Visby’s website helps find the fastest option for users.) The test looks for chlamydia, gonorrhea, and trichomoniasis, but can only be used once.
Anyone who tests positive will be offered a same-day telehealth appointment via the Optum Now network. The telehealth appointment is included in the price of the test. For people who prefer to see their own provider, the app will prepare a PDF of the results that can be sent to a provider via a patient portal or printed out and brought to an appointment.
At-home STI testing has become more popular in recent years because it is private and convenient. There are many kits on the market today that let users collect their own sample, but most of them need to be mailed to a lab for results. Until now, the only tests that offered immediate results at home were an oral HIV test and a test for syphilis that uses a drop of blood from a finger prick.
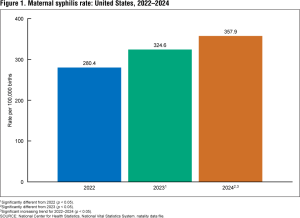
Expanding testing options is important. There were more than 1.5 million cases of chlamydia and over 500,000 cases of gonorrhea reported 2024. In addition, the CDC estimates that at any given time, 2.6 million people trichomoniasis (often called trich), though exact numbers are not known because cases do not have to be reported.
The CDC estimates that there are many more infections each year that go undiagnosed because these STIs often have no symptoms. If diagnosed early, all of these infections can be cured with antibiotics. When left untreated, however, they can cause serious health issues including infertility. That’s why regular testing is so important.
At-home STI testing may help more people get tested because it can be easier to coordinate than in-person testing. It can also be good for people who don’t feel ready to talk about their sexual health with a health care provider. A recent study found that 70% of young people say they would prefer testing for STIs at home over going to a doctor’s office or clinic. Respondents cited convenience, privacy, and comfort as reasons why they preferred STI self-collection kits.
There are now many ways to test at home. Visby’s new test is good at-home option because results are given right away, and telehealth follow-up is included. However, the test is more expensive than other options. There are many other online companies—such as Nurx, TBD Health, Lemonaid, Let’s Get Checked, and myLAB—that sell test kits for STIs including chlamydia, gonnorhea, and trich. Similar tests are also available in pharmacies. Results for these tests take longer because these tests have to be mailed to a lab, but they are less expensive. Some health departments also offer at-home test kits that can be picked up or set by mail. There may also be free at-home testing kits available in some areas.
All of these tests provide accurate results so everyone can pick what option works best for them. The most important thing is to get tested and get any follow up care needed as soon as possible.