FDA Approves New Version of PrEP—Just Two Shots A Year
The FDA has approved lenacapavir as a form of pre-exposure prophylaxis (PrEP), offering a new option for HIV prevention requiring only two shots per year.

According to new data from the Centers for Disease Control and Prevention (CDC), only one in three adults in the U.S. infected with hepatitis C have been cured despite the introduction of direct-acting antivirals almost 10 years ago.
Hepatitis is an inflammation of the liver. It can be caused by a group of viruses—hepatitis A, B, C, D and E. These viruses can damage liver cells and cause scar tissue. Symptoms can range from mild (fatigue) to more severe (mental confusion). In many cases hepatitis is not a serious threat to health and can resolve on its own. For some people, though, the disease can become long-lasting and may lead to liver failure and death.
Hepatitis C virus (HCV) is primarily transmitted by direct contact with blood. It can be transmitted through sharing needles or other injecting equipment during intravenous drug use. Having an STI or HIV, having sex with multiple partners, or engaging in rough sex may increase the likelihood that a person will contract HCV.
Until about 10 years ago, treatment for HCV involved versions of interferon. These treatments often came with unpleasant side effects. They were also not considered a cure, as a significant percentage of patients relapsed.
In 2013, Gilead introduced Solvadi, a 12-week course of treatment that cured HCV in up to 96% of patients. While this was a major breakthrough, people were outraged by the $84,000 price tag which prompted a Congressional investigation. Today, there are a number of similar medications that are nearly 100% effective in curing HCV. This includes lower-priced generic versions, but even these can cost over $20,000 without insurance.
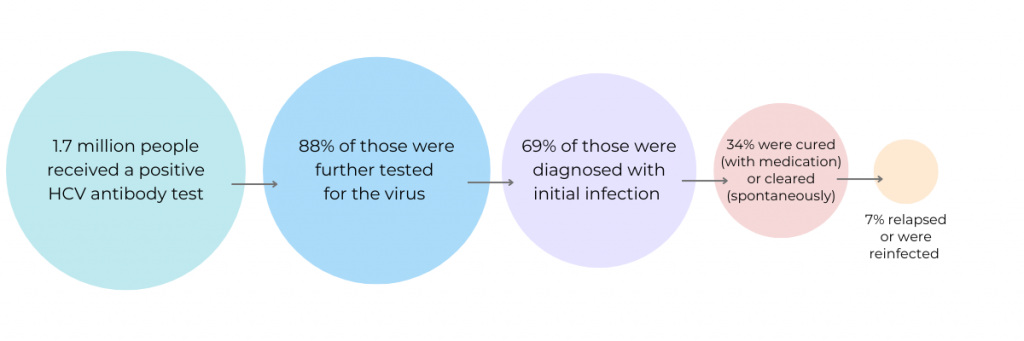
A new study published in Morbidity and Mortality Weekly Report analyzed data from patients tested for hepatitis C. Over 1.7 million patients who had received a positive HCV antibody test were included. Of those that had an initial infection and were eligible for treatment, 34% were cured (with medication) or cleared (spontaneously). Among this group, however, 7% were later found to have a persistent infection or reinfection.
The researchers then looked at data by age, sex, and type of insurance. Not surprisingly, patients with other payor, unspecified payor/insurance, or Medicaid had lower viral clearance (23%, 33%, and 31%, respectively) than those with Medicare and commercial insurance (40% and 45%, respectively).
At a White House briefing on the topic, lead research Carolyn Wester called the cure rates “jarringly low.” She suggested the high price of treatment and numerous restrictions put in place by insurance companies were partially to blame. Some payers limit which patients are eligible for treatment. They may also require burdensome pre-authorization before treatment can begin. Insurers may even limit the types of providers who can prescribe treatment. All of these restrictions can delay or even prohibit access to this life-saving medication.
Other experts pointed point to the cumbersome testing process. The two-step process requires patients to get their results and then come for a second confirmatory test. This can lead to delays in treatment for some people or no treatment for those who don’t follow up. (The CDC recommends one-time hepatitis C testing of all adults 18 years and older. It also suggests that all pregnant women get tested during every pregnancy.)
Francis Collins, who is leading the White House National Hepatitis C Elimination Program, addressed this at the briefing. He argued that the U.S. needs rapid point-of-care tests like those already available in Europe and Australia. Collins suggested that initiating a rapid testing program could save the $44 billion over the next 20 years. It’s rare to have both an opportunity to save lives and save money, but that’s what we have here. You can’t know this and just walk away, he said.
The Viral Hepatitis National Strategic Plan calls for more than 80% of people with hepatitis C to achieve viral clearance by 2030. This study shows that we are still far from reaching that goal.

The FDA has approved lenacapavir as a form of pre-exposure prophylaxis (PrEP), offering a new option for HIV prevention requiring only two shots per year.

On a recent episode of Love Island, a cast member sugested that we could blame our current STI epidemic on men who had sex with animals. She pointed to koalas with chlamydia as an example. There’s some truth here, but also a lot of misinformation.

A new report from the Centers for Disease Control (CDC) shows that we’re missing opportunities to prevent congenital syphilis and save lives.
There’s potential good news in gonorrhea prevention as a series of studies suggests that certain meningococcal B (MenB) vaccines can reduce the risk of gonorrhea.
There is new guidance on pain management for IUD insertion and acknowledgement that providers often underestimate the pain patients feel during their procedures.

The FDA just approved the Teal Wand, a self-collection device for HPV testing that does not require a speculum exam or even a trip to the doctor’s office. People can collect their own sample at home and send it to a lab for analysis.
ASHA believes that all people have the right to the information and services that will help them to have optimum sexual health. We envision a time when stigma is no longer associated with sexual health and our nation is united in its belief that sexuality is a normal, healthy, and positive aspect of human life.
ABOUT
GET INVOLVED
ASHA WEBSITES
GET HELP
© 2025 American Sexual Health Association