The Department of Health and Human Services (HHS) recently updated the Sexually Transmitted Infections National Strategic Plan for the United States: 2021–2025 to include an addendum on herpes (HSV). HSV is widespread in the United States. An estimated 18.5 million adults are infected with HSV-2, which primarily causes genital herpes. It is also estimated that 48% of people ages 14 to 49 have HSV-1 which can cause either oral or genital herpes.
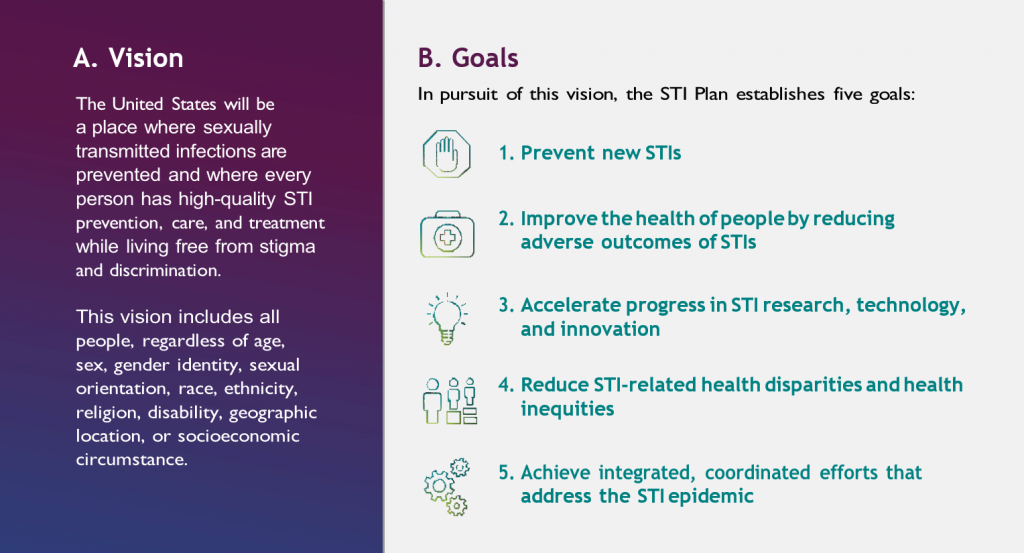
The STI Plan, first released in 2020, sets out a roadmap for preventing and controlling four of the most common STIs: chlamydia, gonorrhea, syphilis, and human papillomavirus (HPV). HSV was not originally included. In 2022, Congress directed HHS to amend the plan to address the prevention and treatment of herpes.
There are many challenges that come with preventing and treating HSV.
- It is highly contagious and already widespread. Herpes is passed from skin-to-skin contact. Anyone who has oral, anal, or vaginal sex can get HSV-1 or HSV-2.
- It is a lifelong condition. There are treatments for herpes, but there is no cure. For most people herpes symptoms are very mild. Some people will only ever get sores once, while others will have “outbreaks” throughout their lives.
- It can be hard to know if/when you’re contagious. People with herpes are most contagious when sores are present. Not all sores are visible, however, and some can be mistaken for other skin issues like an ingrown hair. People with HSV may also experience “asymptomatic shedding.” This is when virus cells are on their skin, but they don’t have any symptoms. HSV is contagious during this time.
- There are few prevention methods for sexually active people. There is no vaccine for HSV, and no biomedical methods like Doxy PEP for bacterial STIs or PREP for HIV. Condoms are an effective tool to prevent transmission of genital herpes, but herpes sores can occur in areas that are not covered by a condom, like on a person’s scrotum or butt. Condoms might also not cover areas affected by asymptomatic shedding.
- Regular screening for HSV is not recommended. Herpes is easy to diagnose during an outbreak. Health care providers can swab lesions, and tests can determine if the sores are caused by HSV-1 or HSV-2. Testing when no sores are present is more complicated. There are blood tests that can look for the antibodies your immune system creates when exposed to HSV, but these tests are not highly accurate. Because of these testing challenges, the United States Preventive Services Task Force does not recommend widespread screening for herpes. Instead, the Task Force recommends that individuals get tested if they have potential symptoms or have reason to believe they were exposed.
- Many people with HSV don’t use medication to suppress outbreaks. Antiretroviral medications can suppress the virus. This can reduce the number of outbreaks someone has and the severity of symptoms. It can also reduce asymptomatic shedding and the risk of transmission. In one study, however, only 29% of HSV patients reported receiving this medication. Most people did not think their outbreaks were severe or frequent enough to need treatment.
- There is a lot of stigma around HSV. HSV is a lifelong condition caused by sexual activity. Unfortunately, some people still believe that having an STI, herpes in particular, is a sign of promiscuous or irresponsible behavior. Herpes sores are also still seen by some as a sign of being “dirty.” People diagnosed with genital herpes often experience anxiety, depression, and low self-esteem. They may worry about being rejecting by their current or future sexual partners. Health care providers are often not as sensitive to these fears as they should be.
The HSV Addendum integrates the latest science in HSV diagnostics, prevention, care, and treatment into the STI Plan in the hopes of overcoming these challenges.
It also prioritizes federal action steps in each of these areas and identifies the agencies responsible for each. These action steps are integrated into the five goals of the STI plan which including preventing new infections, reducing the adverse outcomes of STIs, accelerating STI research and innovation, reducing STI-related health disparities, and coordinating efforts to address the STI epidemic.